The article published in Minerals written by: Justyna Ciesielczuk, Mateusz Dulski, Janusz Janeczek, Tomasz Krzykawski, Joachim Kusz and Eligiusz Szełęg “Crystal Chemistry of an Erythrite-Köttigite Solid Solution (Co3-xZnx) (AsO4)2·8H2O” was awarded by the Minerals editors as Featured Paper in Issue 7, Volume 10, 2020.
Natural cobalt (erythrite) and zinc (köttigite) arsenates are ubiquitous in the weathering zones of metal deposits. These minerals may have mixed chemical compositions due to easy exchange of zinc and cobalt ions in their structure, as found in the synthetic equivalents of these minerals. However, the continuity of this ion exchange in nature, i.e. the existence of a complete solid solution of erythrite and köttigite in nature, has not been demonstrated so far. Analysing numerous samples from the unique polymetallic oxidation zone of the deposit in Miedzianka in the Sudetes and performing 656 microprobe analyses supported by research on the structure of crystals of mixed chemical composition using X-ray diffraction and Raman spectrometry, the authors proved the existence of a solid solution: erythrite-köttigite with 90% miscibility, which outermost parts have the composition (Co2.84Mg0.14Zn0.02) (AsO4)2·8H2O and (Zn2.74Co0.27) (AsO4)2·8H2O. The sequence of crystallization in the erythrite-köttigite solid solution from the cobalt-rich member to the zinc member reflects the chemical evolution of the solutions towards higher acidity associated with a gradual increase in Zn (II) activity and Co (II) depletion during erythrite precipitation.
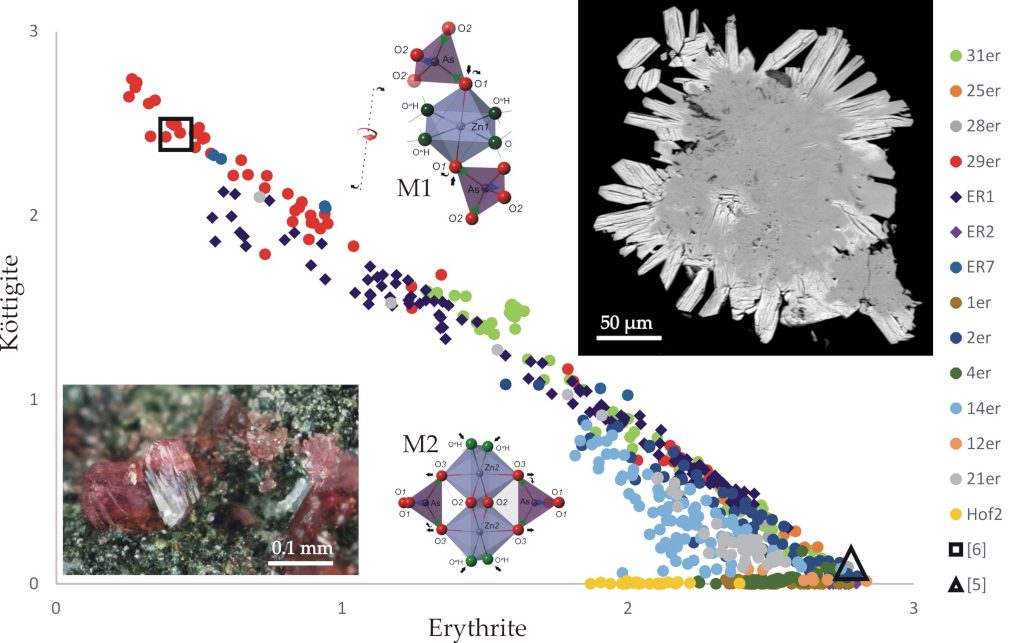
The refined structure of the crystal with the composition Zn/Co = 1.78 showed a structure of the vivianite type with a disordered arrangement of zinc and cobalt ions in octahedral positions. During microprobe analysis, the köttigite structure was found to be unstable in vacuum and more susceptible to electron beam-induced dehydration than the erythrite structure, due to the instability of Zn ions in octahedral coordination.
While isomorphic substitutions of cobalt with Zn, Fe, Ni, Mg in erythrite are possible, köttigite practically does not accept other elements other than Co into its structure. Pure köttigite was not formed in the Miedzianka deposit, while zinc arsenate of an unknown structure was identified. Currently, the authors are working on its identification.
Earlier, the authors proved the formation of expanded solid solutions in the Miedzianka deposit between copper and zinc arsenates and phosphates: 77.5 mol% cornwallite-pseudomalachite and 75 mol% kipushite-philipsburgite (Ciesielczuk J., Janeczek J., Dulski M. & Krzykawski T. Pseudomalachite–cornwallite and kipushite–philipsburgite solid solutions: chemical composition and Raman spectroscopy. Eur. J. Mineral. 2016, 28, 555–569).





