On 6 June 2025, Chemist Day is celebrated. It is a movable holiday that falls each year on the first Sunday of June. On this occasion, we wish all chemists and passionate chemists the very best!
For chemistry, you have to feel the chemistry. The decision to choose this path must evoke a whole range of reactions: interest, delight, surprise…. We asked our chemists why they chose this particular field of science.
„Save the date” is a series of articles that have been written to celebrate various unusual holidays. The authors of the presented materials are students, doctoral students and employees of the Faculty of Science and Technology of the University of Silesia.
Does chemistry still exist?
The Nobel Prize in Chemistry awarded to computer scientists for a breakthrough in biology
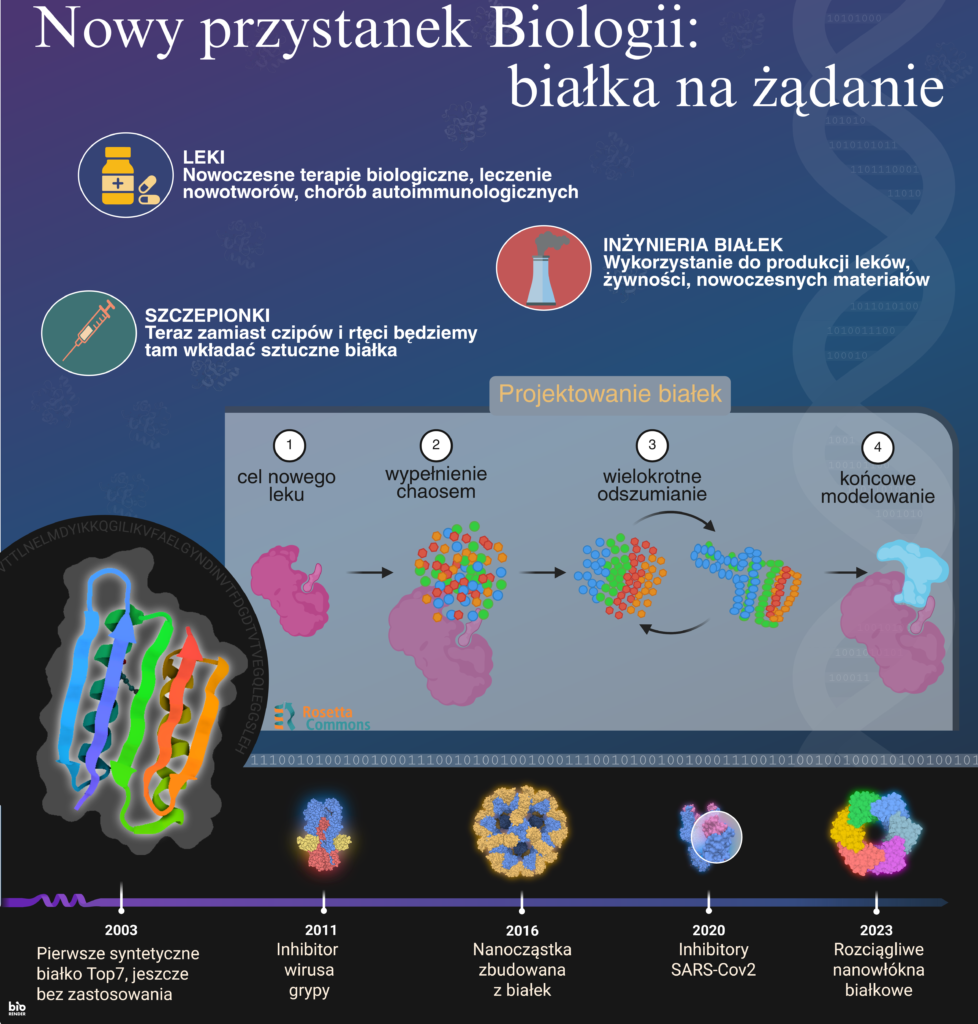
In 2024, the Nobel Prize in Chemistry was awarded to David Baker, John Jumper and Demis Hassabis. It is truly a crowning (but not concluding) achievement of one’s scientific career. However, if these three scientists had decided to work at an average chemistry faculty in Poland, their careers could have ended before they even began. It sounds absurd, but in the current system of assessing scientific achievements it is unfortunately quite easy to imagine. Their publications, although cited by thousands of scientists around the world, would probably not be included in the appropriate “specialisation basket”. Not many points. Discipline not entirely consistent. In addition, there is no justified profile of scientific activity as part of the faculty development strategy. These guys seem more interested in computer games! If they were just starting their scientific careers, they probably would not even get into a doctoral school. In our current reality, it is not discoveries, but points that decide whether you are a “good scientist”. And points are earned not for pushing the limits of knowledge, but for fitting into a given rubric. And if the rubrics show clear signs of manipulation… you can probably figure out on your own where we find ourselves. So, if one of the Nobel Prize winners tried to apply for a job, promotion or grant, he might have been asked: “But tell me, where exactly do we see chemistry here?*” And his career would probably end before it even began. I say this with full awareness and experience of a person who has been operating in this system for years and sees every day how much it does not fit the reality of modern science.
But let’s be fair—science, like any other area of life, must be subject to some supervision and management. After all, these are public funds, and the public has the right to expect that they are spent wisely. No wonder this requires systems, classifications, points, rankings… The problem arises when we try to manage science as if it were an assembly line—one system for all cases. Not every mechanisation solution is suitable for packing tomatoes, and even more so not every strategy of this kind is suitable for assessing research work, which by definition escapes rigid frameworks. We understand this first example immediately when we look at the squashed vegetables in plastic trays. However, the latter—that something valuable may not fit into the established system—is difficult to accept when points, slots and numbers fill the tables so beautifully. And if there is one thing every official is afraid of, it is not someone asking them one day whether they have distributed the funds at all, but why have they distributed them in such a stupid way.
Okay, now we can ask again where is the place for chemistry in all this? Because, of course, last year’s Nobel Prize in Chemistry makes a lot of sense. And this sense goes beyond the fact that AI is trendy and proteins are important. The point is that this story tells us something fundamental about what chemistry is today, and what it must be if it is to continue to respond to the real challenges of the world. Protein design is, after all, pure chemistry—but before one laboriously synthesises the molecule in the flask, they design it on a computer. And predicting its structure using AI? It is nothing more than the development of structural chemistry—the same chemistry that was once based on crystallography and physical models and today goes beyond them. Today we do it faster, more precisely, using tools that we did not have before. And it is this molecular purpose and approach, not form, that keeps us at the heart of chemistry. This prize was not awarded despite the fact that the research was cross-disciplinary but precisely because of it. What was honoured was not only the creators of a specific tool (AlphaFold) or method (protein design), but a certain way of thinking: brave, boundary-crossing, one which allows us to look for answers where no one had looked before. But to understand it, we need to be able to break out of the comfortable but tight framework that we impose on ourselves. And we have a serious problem with this in Poland.
We still operate in a system in which it is better to do safe, predictable research than to risk something truly innovative; in which grants are distributed according to disciplines assigned ex officio, not according to the essence of the problem; in which a young scientist often has to choose: whether to do something interesting or something that “scores points”. And these are not isolated cases—this is the everyday life of many great researchers who are slowly being pushed out of science by the system. This is also a problem of lack of funds resulting in an approach where potentially unsuccessful, risky projects become synonymous with “a waste of money”.
What’s worse is that this way of thinking starts much earlier. At school level, we teach chemistry, biology and computer science as three separate worlds. Teachers rarely have the space to show that they can interpenetrate. And when we do come up with the idea that they can be taught holistically, we vaguely call this subject “science” and we start the process of discouragement from the top. A student who is interested in AI and proteins is often deemed too “biological” for computer science and too “digital” for chemistry. And then they get discouraged and go on to study psychology to understand what is “wrong” with them. If we do not start teaching interdisciplinary thinking based on the exact sciences, do not change the rules for assessing scientific work and do not give young people space to take risks, we will not only not receive the Nobel Prize, but we will simply remain in the technological Middle Ages.
*Exactly the same question, “But tell me, where exactly do we see chemistry here”, was asked to the author of this text and his PhD student during the presentation of their research—which, as it turns out, puts them in quite a respectable group. Will it turn out that every Nobel Prize begins with a misunderstanding? One can always dream 😉
What exactly is AlphaFold and why is it so important?
AlphaFold is an artificial intelligence algorithm created by the DeepMind team, which in 2020 solved a problem that scientists had been puzzling over for decades: predicting the three-dimensional structure of a protein based on its amino acid sequence. Sounds horrendously technical? Then let’s try differently: if a protein is a necklace of beads (amino acids), AlphaFold can predict how this necklace will fold in space—with an accuracy almost equal to laboratory experiments. And the shape of a protein is not a trivial matter—it determines whether it will bind a drug, trigger a chemical reaction or cause a disease. Until now, predicting protein structure was like solving a Rubik’s Cube in the dark—slow, expensive, full of trial and error. AlphaFold has changed this dramatically: in a few minutes it can provide the structure of a protein that experimental laboratories would spend months working on. A Nobel-worthy discovery turned out to be… reversing the entire process. If you can predict what a protein will look like by knowing its structure, you can also design it from scratch, targeting specific properties. The process borrows heavily from computer photo denoising. Relatively simple but time-consuming calculations can remove ugly grainy hues from photos taken in poor lighting. Similarly, the arrangement of building fragments around an important target can be improved to obtain a protein that reacts with that target. Such target can be, for example, a biological-chemical blocker of the influenza virus, or SARS-Cov2, or a protein that has better strength than Kevlar. And all this during sessions with artificial intelligence, instead of long months in the laboratory.
As a consolation for all those who are terrified by the vision of such powerful artificial intelligence, it is worth recalling the story of how a group of experienced gamers, enthusiasts and nerds who do not necessarily have deep knowledge of molecular biology discovered the structure of a protein found in the Mason-Pfizer monkey virus. It is a virus from the so-called immunocompromised retrovirus group, very similar to the human HIV virus. The online game Foldit allowed players to freely adjust protein fragments to obtain the lowest energy and therefore the highest probability that it was the correct arrangement. Kind of like Tetris but with atoms. This is where the human desire to play and learn, as well as the innate curiosity that makes us wonder: “what would happen if…?” ended up yielding better results than the efforts of many scientists using more traditional methods of calculation. Needless to say, one of the creators of Foldit is Prof. David Baker.