Evolution equipped living organisms with various means of reproduction. One of them is parthenogenesis observed in some of the representatives of the animal world. It turns out to be quite an exciting way to optimise the costs associated with sexual reproduction.
Contact
Prof. Piotr Świątek from the Institute of Biology, Biotechnology and Environmental Protection of the University of Silesia – piotr.swiatek@us.edu.pl
Parthenogenesis In Aphids, Bees And… Humans?
| Małgorzata Kłoskowicz, PhD |
How is it possible that a virgin queen, which was unable to fly out of the hive for her first and only mating flight, laid unfertilised eggs? What’s more: viable males, i.e. drones, hatched from the eggs. This was the question that Rev. Jan Dzierżon, a beekeeper living at the turn of the 20th century, an enthusiast and inventor of the prototype of the modern hive, had to ask himself when he first observed virgin birth in bees.
We now know that natural parthenogenesis (virgin birth) is observed not only in insects and many other invertebrates but also in fish, amphibians, reptiles, and birds. It does not occur in mammals, although, as experiments have shown, it can be artificially induced in mice. In humans – not yet.
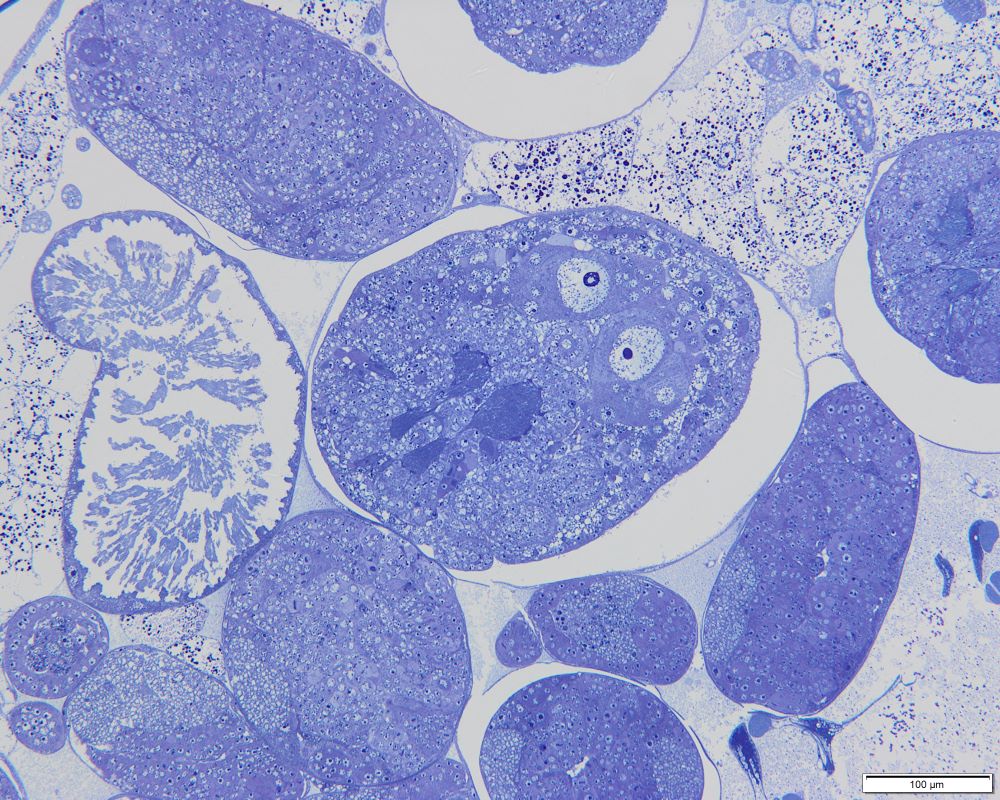
The insides of the parthenogenetic aphid Cinara cupressi larvae, with numerous embryos of the next generation of virgin females. Parthenogenetic aphids do not lay eggs, but give birth to daughters that already contain developing granddaughters | Photo: Piotr Świątek, Karina Wieczorek
Nature’s Outrageous Experiment
Everything that happens in nature aims at survival. If nature experiments from time to time, it is only so that those who reproduce faster and more efficiently have better chances of surviving. When viewed through the lens of sexual processes, parthenogenesis is just such an experiment, allowing nature to optimise the costs associated with sexual reproduction under certain conditions. Cost-effectiveness comes first!
The first of considerable expenditures is the males’ cost of living. From the perspective of the survival of a species, their only role is to provide half of the necessary genes. Male gametes are not only cheap but also produced in excess. Thus, a single male capable of impregnating many females should be sufficient. Meanwhile, we have equal representation of both sexes in nature, which means that only 50% of the population reproduces. Given that the primary objective is survival, this is waste in its purest form.
The second expense incurred by sexual reproduction is the cost of meiosis, which necessitates sharing our genes with a sexual partner in order to have offspring. We would not be able to clone Maria Skłodowska-Curie, for example. Her children got only half of their mother’s genes and half of their father’s genes, so they are different from each of their parents. Sexual reproduction must therefore result in the mixing of genes in our offspring. And thus we return to the beginning. Scientists searched for an answer to the question of why good gene arrangements are broken up. They came to the conclusion that mixing them gives us an advantage by ensuring better chances of survival. In sexual reproduction, each offspring is different. The one better adjusted to the given conditions will survive and reproduce, whereas the one worse suited will not reproduce and will become extinct.
Is this a satisfactory explanation for the existence of the costs associated with sexual reproduction? It is difficult to say. In nature, things are usually much more complicated than they seem.
So let us take a look at some situations where nature departs from sexual reproduction. Another option could be parthenogenesis or hermaphroditism (common among plants). To put it in the most general terms, parthenogenesis is reproduction without the participation of males and, consequently, without conception. However, when we look at it more closely, we find a plethora of solutions. We already know of more than a dozen recipes that nature has discovered for obtaining successive generations of daughters and sons without ‘fathers’. In the case of invertebrates and certain vertebrates, it is presumably associated with higher chances of survival and optimisation of the costs associated with sexual reproduction under certain environmental conditions.
Bdelloid rotifers are an evolutionary outlier in this regard, or a downright evolutionary scandal, at least according to one of the most prominent experts in evolutionary biology, Prof. John Maynard Smith. Model-based calculations suggest that after about 10,000 generations, a species reproducing in such a way should have become extinct. Meanwhile, female bdelloid rotifers never laid eyes on a male for hundreds of millions of years, yet they exist everywhere in the world and are doing just fine.
The first documented evidence of parthenogenesis appeared as early as the mid-18th century. Charles Bonnet, a Swiss philosopher and naturalist interested in aphids, observed that some females give birth to successive generations of females without copulating. Daughters give birth to daughters that give birth to daughters. This is a perfect example of the combination of rapid colonisation and survival. Anyone who has once encountered them on their plants knows how quickly they can colonise any green space.
Aphids reproduce both sexually and without the involvement of males. Their fertilised eggs are laid in the winter, which is how the species is able to survive the harshest time of the year. In the spring, females (referred to as fundatrix or foundresses) hatch from the eggs. A single female searches for a host plant. When she comes across one, she rapidly begins to colonise it, giving birth to daughters, which are her clones. There is already another embryo in the nascent larva, making the females resemble Russian dolls. Their numbers are increasing exponentially. When the autumn ends, everything changes. Aphids start laying two kinds of eggs. Females hatch from one type and males from the other. This is the generation that will reproduce sexually, the resulting eggs are prepared for overwintering, and the whole process repeats itself.
Bees have a slightly different approach to survival. The young queens fly out of the hive for a mating flight only once in their entire lives. They come into contact with at least one drone and, as a result of this encounter, they accumulate sperm in special sacs. They return to the hive and lay the eggs fertilised in this way, which then give birth only to females — up to several tens of thousands of sisters forming a single swarm. When the supply of sperm runs out, then reproduction without males takes over. Parthenogenesis in bees involves the laying of haploid unfertilised eggs, which always hatch into drones.
Oleander aphid Aphis nerii | Photo: Wirestock — Freepik.com
Humans’ Outrageous Experiment
The great conundrum of parthenogenesis was primarily about how to stimulate the egg cell. In sexual reproduction, this process is initiated by the sperm. It is not without reason that the metaphor of the kiss of the sleeping beauty is used, which in this case involves the inactive oocyte. The sperm transmits an electrical and enzymatic impulse and stimulates the egg cell. Scientists concluded that some animals must produce another stimulus triggering parthenogenetic reproduction. At the end of the 19th century, biologists performed a series of experiments to understand this phenomenon. They pricked the egg cell, rubbed it with various instruments, such as a glass pipette, and used a salt solution to see how the stimulation occurs. Interestingly, these actions resulted in embryos actually developing, although very rarely.
Scientists also wondered why natural parthenogenesis does not occur in mammals. The biologists have concluded that the so-called parental genomic stigma (genomic imprinting) may be the cause. They discovered that there is a molecular mechanism that prevents the organisms of mammals from reproducing without male participation. The DNA supplied to the offspring by the mother and father is marked differently (by the addition of methyl groups) according to a sex-specific pattern. In mice, it has been shown that an embryo develops only when one specific gene has the stigma of the mother and the other of the father. Only the interaction of the two genomes can ensure the viability of the offspring. Nature has thus protected mammals from escaping the high cost of sexual reproduction.
Despite this, intensive research using knowledge from molecular biology began in the 20th century to bring about artificial parthenogenesis in mammals. The results of successful experiments were published in 2004. Scientists from Japan found a solution to ‘bypass’ the protection mechanism — they reprogrammed one of the female genomes into a male one. Out of one hundred mouse embryos, created by combining the genetic material of two females, only two survived. The rest had many serious developmental defects. Just one single embryo made it to adulthood and was fertile. The mouse was given the name Kaguya.
This type of research evokes all sorts of emotions and raises many questions. Could artificial parthenogenesis also be carried out in humans? What consequences and ethical justification would there be for this type of experimentation? It would not be possible to avoid associations with factories mass-producing genetically modified humans. If we could legally edit genes in the future, we would be able to design improved clones of ourselves. We could get rid of gender, race, and perhaps our bodies. Daughters could give birth to daughters and virgins to males. But would males still be needed?
The article entitled ‘Daughters Birth Daughters, Virgins Birth Sons’ was published in the popular science journal of the University of Silesia No Limits no. 1(7)/2023.





